The Surprising Link Between Splicing Errors and Longevity
2025-12-03 15:24
Keywords: spliceosome fidelity, intron retention, mTORC1 signaling, longevity, rnp-6, egl-8
Introduction
In the scientific exploration of aging and longevity, RNA splicing, which is a fundamental process in molecular biology, has increasingly become a focus of research. RNA splicing is a critical step in the regulation of gene expression, generating mature mRNA through the removal of introns and the ligation of exons. The spliceosome is the complex molecular machinery responsible for this process, and alterations in its fidelity are closely linked to the loss of cellular homeostasis and aging. However, only a few splicing factors have been demonstrated to have a causal effect on lifespan extension, and the underlying mechanisms and downstream targets remain unclear. A study published in Nature Aging has revealed an exciting new mechanism: reduced spliceosomal fidelity and the retention of an intron in the egl-8 gene can extend lifespan by suppressing the mTORC1 signaling pathway.
Construction of SunyBiotech
SunyBiotech has engineered a series of strains to investigate the regulatory role of intron retention in aging:
PHX645 rnp-6(syb645) and PHX626 rnp-6(syb626):GFP-tagged RNP-6 strains
PHX1074 rbm-39(syb1074):rbm-39(S294L) mutant strain
PHX3661 egl-8(syb3661):mNeonGreen-tagged EGL-8 strain
PHX4850 egl-8(syb4850):egl-8 intron 8 3’-splicing site-edited strain
PHX1527 rbm-39(syb1527) and PHX1545 rbm-39(syb1545):mKate2-tagged RBM-39 strains
1. Spliceosomal fidelity and longevity
In this study, researchers identified a hypomorphic mutation in the spliceosomal component RNP-6 (PUF60), which led to aberrant splicing, enhanced stress responses, and significantly extended the lifespan of Caenorhabditis elegans (Fig. 1A and 1B). Through a genetic suppressor screen, researchers also discovered a gain-of-function mutation in the splicing factor rbm-39, which interacted with RNP-6. This mutation increased nuclear speckle formation (Fig. 1E), alleviated splicing defects (Fig. 1D), and suppressed the lifespan extension induced by the rnp-6 mutation (Fig. 1C). This finding indicates that the gain-of-function mutation in RBM-39 partially counteracts the longevity effects of the RNP-6 mutation.
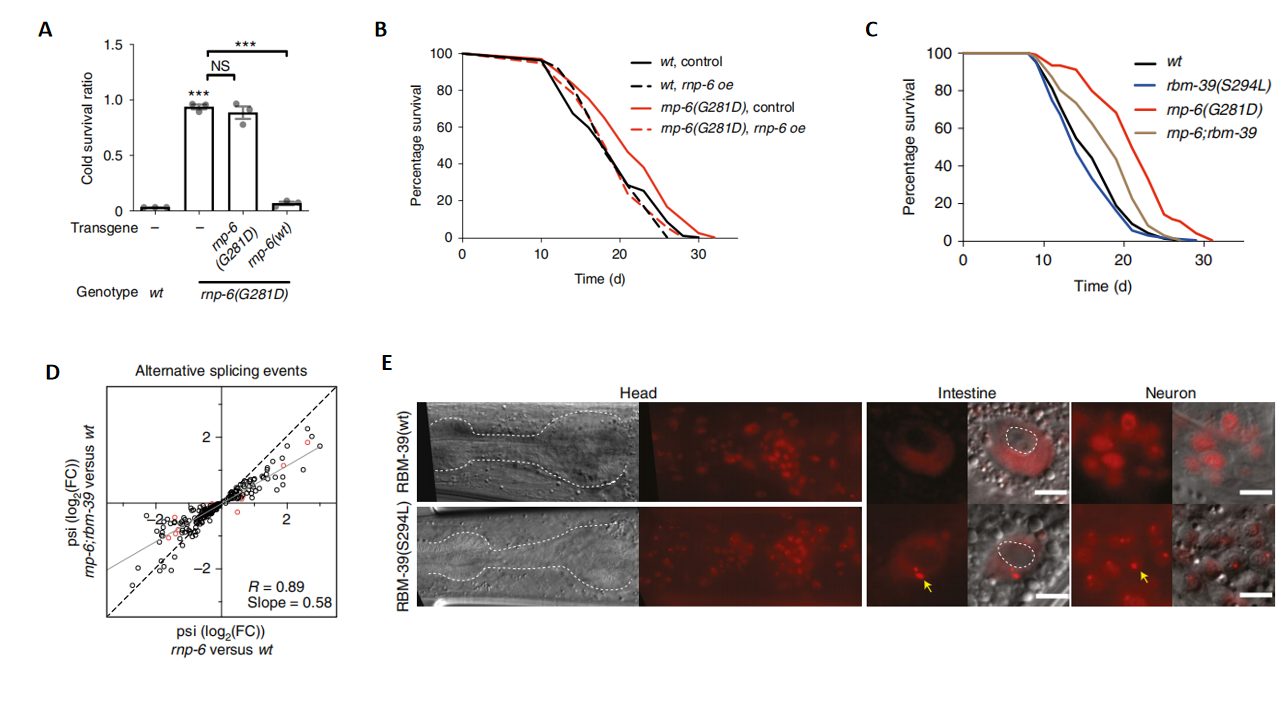
Figure 1. Spliceosomal fidelity and longevity
2. The key role of egl-8 intron retention
Further investigation revealed that the retention of an intron in the egl-8 gene is a key target among the splicing changes induced by RNP-6/RBM-39 activity, and this intron retention contributed to lifespan extension (Fig. 2A), indicating that splicing changes in specific genes play a critical role. Specifically, the retention of the eighth intron in egl-8 introduced a premature termination codon, leading to mRNA degradation or the production of a non-functional truncated protein (Fig. 2C). In rnp-6 mutant worms, the expression level of egl-8 was significantly reduced in neurons, and restoring normal splicing of egl-8 recovered its expression level (Fig. 2B). These findings suggest that rnp-6(G281D) promotes lifespan extension by retaining the egl-8 intron in the nervous system.
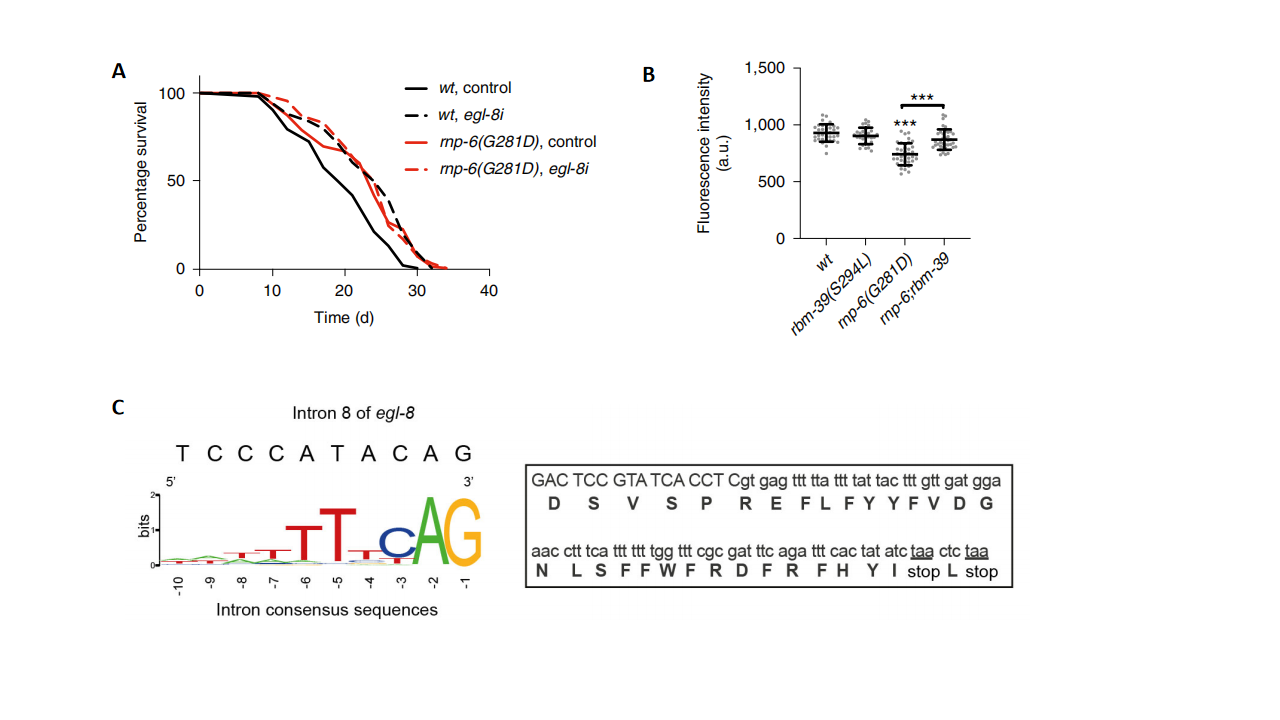
Figure 2. The key role of egl-8 intron retention
3. Regulation of the mTORC1 signaling pathway
mTORC1 is a key regulator of cell growth and metabolism, and its activity is closely associated with aging and lifespan. The study found that the RNP-6/EGL-8 axis primarily functioned in the nervous system to extend lifespan by downregulating the mTORC1/RAGA-1 signaling pathway (Fig. 3A-3D). In human cells, downregulation of PUF60 also strongly and specifically suppressed mTORC1 signaling. The specific mechanism included the relocalization of mTOR from lysosomes (Fig. 3E and 3F), indicating an evolutionarily conserved role for PUF60 in regulating the mTORC1 signaling pathway.
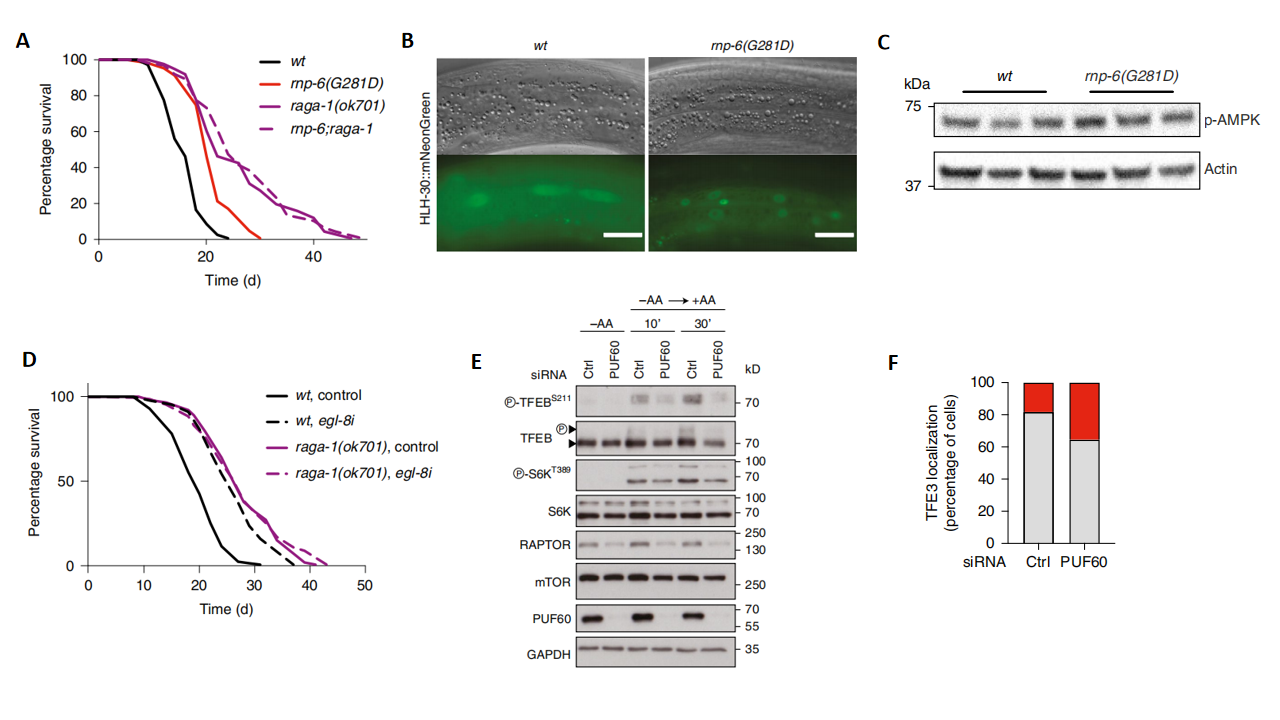
Figure 3. Regulation of the mTORC1 signaling pathway
Conclusion
This study reveals a novel mechanism by which the spliceosomal complex regulates lifespan through intron retention and identifies potential targets for healthy aging interventions. Precise targeting of PUF60 or RBM39 may yield health benefits comparable to those of rapamycin and its analogs. Furthermore, many disorders associated with spliceosomal dysfunction can lead to growth defects, which may be treatable in the future using mTOR modulators. In summary, this research not only provides new insights into the role of RNA splicing in aging but also offers a scientific foundation for developing novel anti-aging strategies.
Reference
Huang W, Kew C, Fernandes SA, et al. Decreased spliceosome fidelity and egl-8 intron retention inhibit mTORC1 signaling to promote longevity. Nat Aging. 2022 Sep;2(9):796-808.





