In-depth Exploration of the Sensory Neuron Transcriptome: The Mystery of mec-2/Stomatin Splicing
2025-11-13 14:38
Keywords: mec-2/Stomatin, alternative splicing, sensory neurons, mechanosensation, Caenorhabditis elegans
Introduction
In the nervous system, how individual neurons precisely execute specific functions remains one of the central mysteries in neuroscience. Despite sharing an identical genome, neurons achieve functional specialization through post-transcriptional regulation, particularly alternative splicing, which generates a remarkable diversity of protein isoforms. MEC-8/RBPMS, a key RNA-binding protein, regulates the expression of mec-2/Stomatin and is essential for the function of touch receptor neurons. In Caenorhabditis elegans (C. elegans), mec-2 encodes a conserved stomatin-family protein required for the proper function of mechanosensory neurons. Although previous studies have suggested the existence of complex splicing patterns in mec-2, the cell-type specificity of its isoforms, their functional distinctions, and the underlying regulatory mechanisms remain poorly understood. A study published in Nucleic Acids Research has elucidated the complex neuron-specific functions and splicing regulation of mec-2/Stomatin in sensory neurons. This research not only reveals intricate mechanisms underlying neuronal function but also provides a valuable model for understanding how alternative splicing contributes to neuronal diversity and sensory perception.
Construction of SunyBiotech
SunyBiotech constructed several C. elegans mutant strains to facilitate research on mec-2/Stomatin splicing strategies in different sensory neurons:
PHX2112 mec-2(syb2112) MEC-2A::wrmScarlet
PHX3795 mec-2e(syb3795) MEC-2E::wrmScarlet.
PHX4210 mec-2e(syb4210) mec-2a(syb2112) MEC-2A::wrmScarlet MEC-2E::3XFlag.
PHX1764 mec-2(syb1764)
PHX3660 elp-1(syb3660)
1. Neuronal specificity of mec-2/Stomatin gene splicing
MEC-8 regulated the alternative splicing sites of mec-2, resulting in long and short isoforms (Fig.1A and 1C). Cell type-specific RNA sequencing of touch neurons in wild-type and mec-8 mutants revealed that many neurons expressed an atypical short isoform of mec-2/Stomatin (Fig. 1B and 1E). In wild-type touch neurons, the MEC-8 protein primarily regulated the splicing of mec-2, leading to predominant expression of the long isoform (mec-2A). In contrast, touch neurons of mec-8 mutants mainly expressed the short isoform (mec-2B) (Fig. 1D). These results indicate that the splicing of the mec-2 gene is tightly controlled by neuronal type and specific regulatory factors.
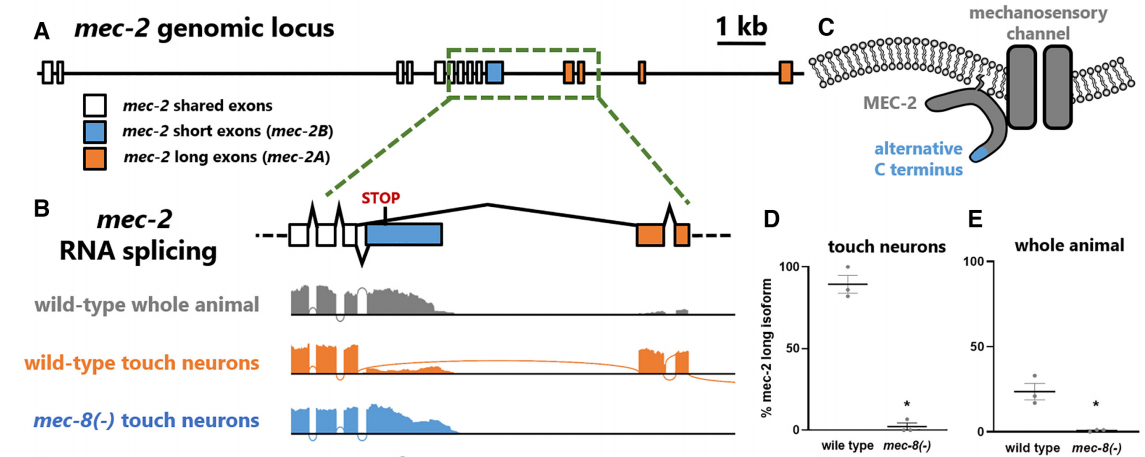 Figure 1. Neuronal specificity of mec-2/Stomatin gene splicing
Figure 1. Neuronal specificity of mec-2/Stomatin gene splicing
2. Functional specificity of mec-2 isoforms
Through the production of distinct isoforms, mec-2 regulates both tactile and olfactory functions. The long mec-2 isoform was the critical variant for mechanosensation, and the short mec-2 isoform cannot functionally substitute for it (Fig. 2D). The short isoform (mec-2B) played a role in olfaction, and mec-2 itself was essential for olfactory function (Fig. 2A-2C). Ectopic expression of the long isoform can effectively substitute for the function of the short mec-2 isoform (Fig. 2E). These findings demonstrate that the different splicing isoforms of the mec-2 gene execute distinct functions in different sensory neurons. Furthermore, in mec-8 mutants, touch neurons failed to correctly express the long mec-2 isoform, leading to defects in mechanical perception. Importantly, genetically restoring the long isoform (mec-2A) completely rescued the tactile perception deficits in mec-8 mutants (Fig. 2F), indicating that the primary functionally relevant target of MEC-8 in touch neurons is mec-2.
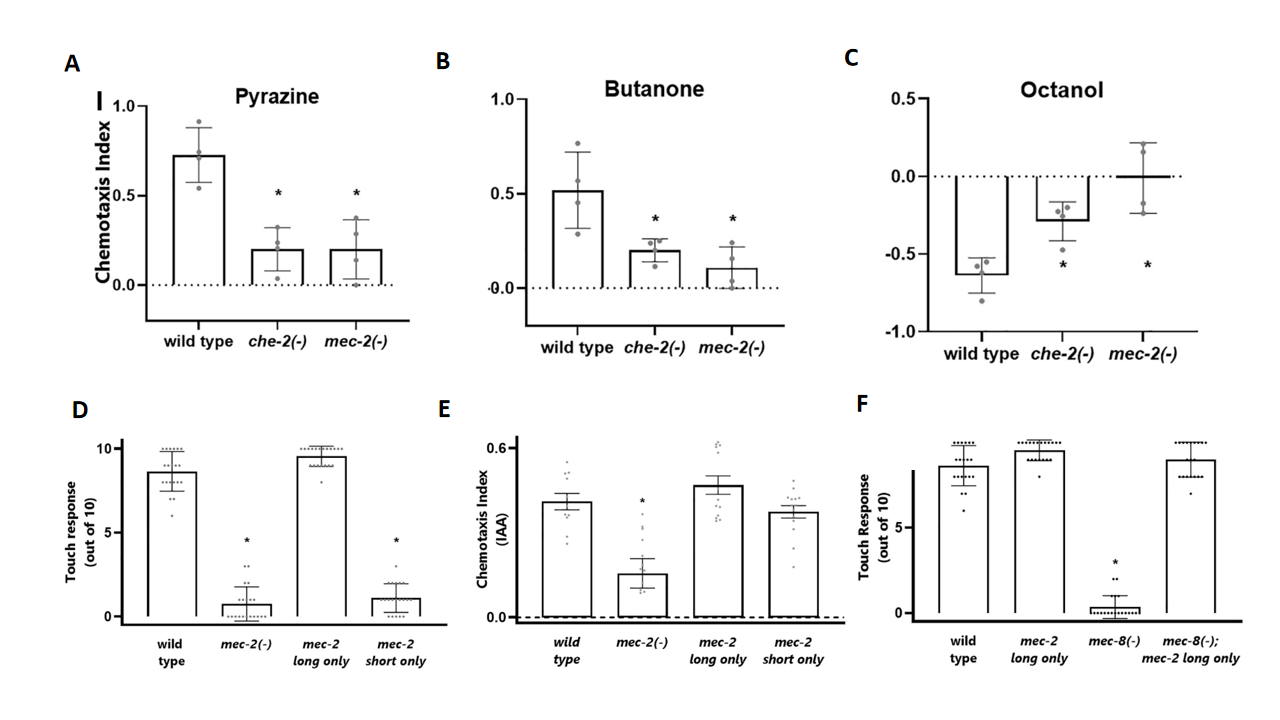 Figure 2. Functional specificity of mec-2 isoforms
Figure 2. Functional specificity of mec-2 isoforms
3. Further splicing diversity of mec-2
Within the long mec-2 isoforms, researchers identified additional subtype diversity. The discovery of a cassette exon further subdivided mec-2 into long and extra-long isoform (mec-2A and mec-2E) (Fig. 3A). These two isoforms were co-expressed in touch neurons, and both must be present simultaneously to restore touch function, as the expression of either one alone failed to reconstitute functional mechanosensation (Fig. 3B). This indicates that they likely function cooperatively, possibly by forming a heteromeric complex.
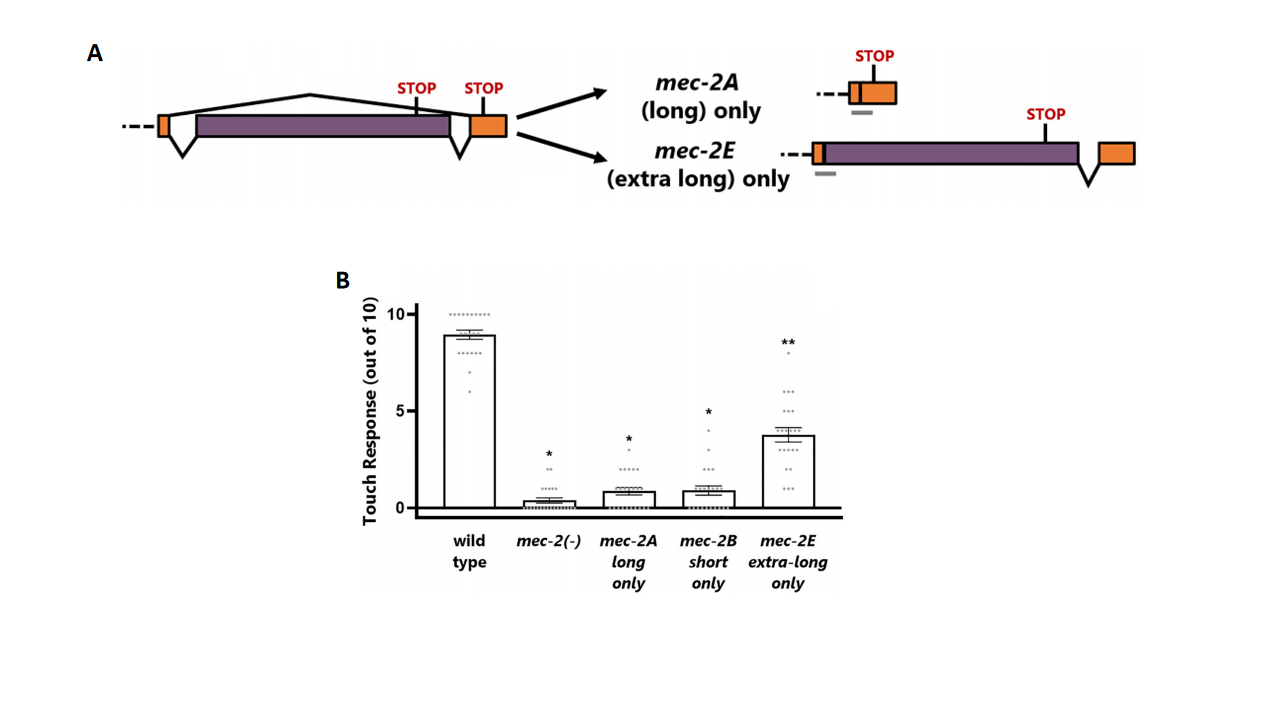
Figure 3. Functional specificity of mec-2 isoforms
Conclusion
This study, through in-depth analysis of sensory neuron transcriptomes, uncovers the neuron-specific functions and regulatory mechanisms of mec-2/Stomatin gene splicing. These findings not only enhance our understanding of neuronal functional diversity but also provide new directions for future neuroscience research. Further exploration of neuron-specific alternative splicing holds promises for better elucidating the complexity of the nervous system and may offer novel insights for the treatment of neurodegenerative diseases.
Reference
Liang X, Calovich-Benne C, Norris A. Sensory neuron transcriptomes reveal complex neuron-specific function and regulation of mec-2/Stomatin splicing. Nucleic Acids Res. 2022 Mar 21;50(5):2401-2416.





