Unveiling the Role of DOS-3 in Stem Cell Aging: Key Insights from SunyBiotech-Supported Research
2024-08-07 16:55
Keywords:
Stem Cell Aging; Germline Stem/Progenitor Cells ; DAF-16; DOS-3 ; Notch Signaling; Caenorhabditis elegans
Contribution of SUNY Biotech
The proximal region of the somatic gonad (PSG) GFP-Expressing Transgene: Ex[Pfos-1a::gfp]Ex[Pfos-1a::gfp]
dos-3 Mutant Allele: dos-3(syb5125)
OLLAS Knock-In Animals: 3xOLLAS::sygl-1
Introduction
Aging is a biological process that leads to the progressive decline of tissue function and regenerative capacity. Understanding the underlying mechanisms of stem cell aging is crucial for developing therapeutic interventions aimed at promoting healthy aging. In a recent groundbreaking study published in Nature Communications, researchers have identified key molecular players that mediate the maintenance of germline stem/progenitor cells (GSPCs) in the nematode Caenorhabditis elegans, providing new insights into stem cell aging and potential avenues for human health applications.
The Study: Objectives and Key Findings
Researchers aimed to elucidate the molecular mechanisms by which the transcription factor DAF-16/FOXO regulates the maintenance of GSPCs in C. elegans. By performing tissue-specific transcriptome analysis, they discovered that DOS-3, a non-canonical Notch ligand, is a direct transcriptional target of DAF-16/FOXO and plays a crucial role in GSPC maintenance.
The study began with isolating PSG cells from daf-2(rf) and daf-16(0); daf-2(rf) mutants. These cells were subjected to RNA sequencing to identify genes differentially expressed under high and low DAF-16/FOXO activity. The RNA-seq data revealed that DOS-3 is significantly upregulated in aging PSG cells of daf-2(rf) mutants, indicating its role in aging-related processes.
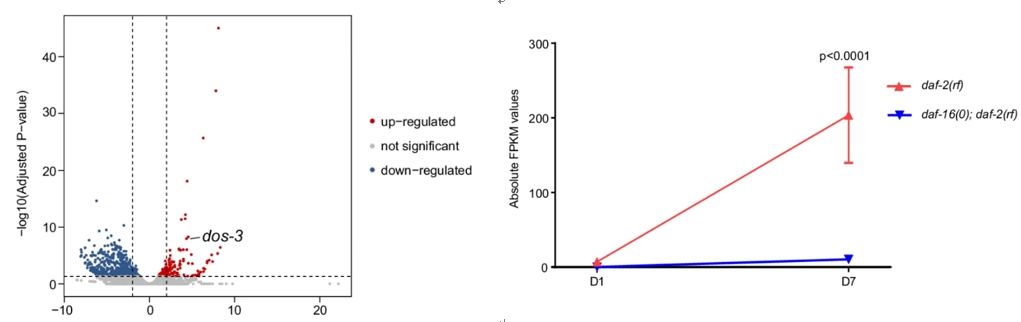
Figure 1: DOS-3 is significantly upregulated in aging PSG cells of daf-2(rf) mutants
Then the author identified consensus DAF-16 binding elements (DBEs) in the promoter region of DOS-3, crucial for DAF-16/FOXO binding. Mutation of these DBEs in the DOS-3 promoter abolished GFP expression in PSG cells of daf-2(rf) animals, demonstrating that DOS-3 is a direct transcriptional target of DAF-16/FOXO.
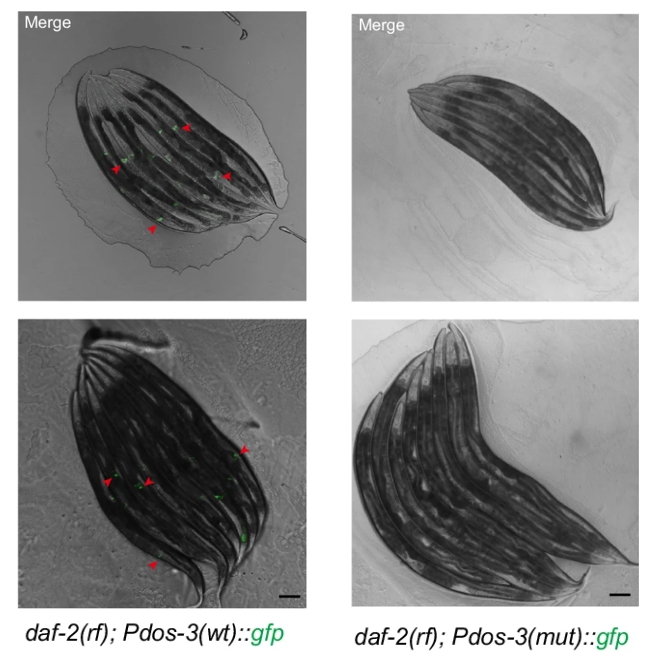
Figure 2: Mutation of DAF-16 binding elements in the DOS-3 promoter abolished GFP expression in PSG cells of daf-2(rf) animals
Mechanism of Action: DOS-3 and Notch Signaling
Further, the study uncovered how DOS-3 functions to maintain GSPCs. DOS-3 activates Notch signaling in the germline, essential for the self-renewal and differentiation of these stem cells. This signaling pathway is vital for preventing the age-related decline in stem cell numbers and ensuring continued tissue regeneration.
The researchers generated a loss-of-function allele of DOS-3, syb5125, using CRISPR/Cas9, showing that DOS-3 is required for maintaining GSPCs in aging daf-2(rf) mutants. Quantitative analysis of GSPC numbers in daf-2(rf) and dos-3(0); daf-2(rf) mutants demonstrated that the loss of DOS-3 leads to a significant reduction in GSPC numbers over time.
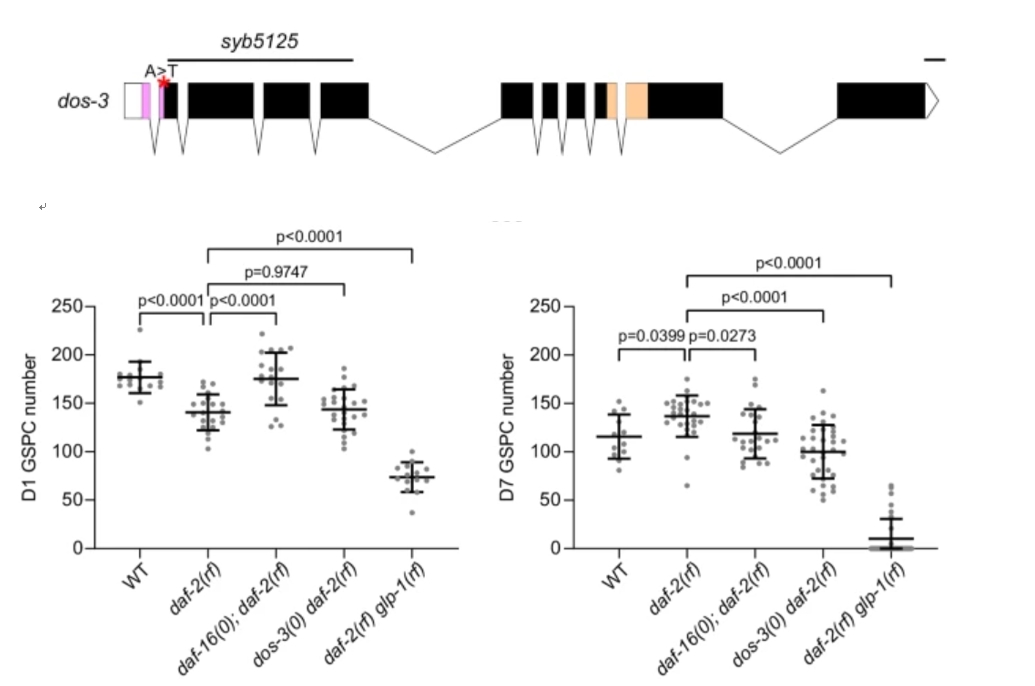
Figure 3: Loss-of-function allele of DOS-3 leads to a significant reduction in GSPC numbers
To figure out how DOS-3 leads to a significant reduction in GSPC numbers. The researchers generated a sygl-1::OLLAS fusion protein to visualize Notch signaling in the germline. Notch signaling in the distal germ line of aging daf-2(rf) animals. Notch signaling was significantly attenuated in dos-3(0); daf-2(rf) mutants compared to daf-2(rf) animals, confirming that DOS-3 activates Notch signaling to maintain GSPCs.
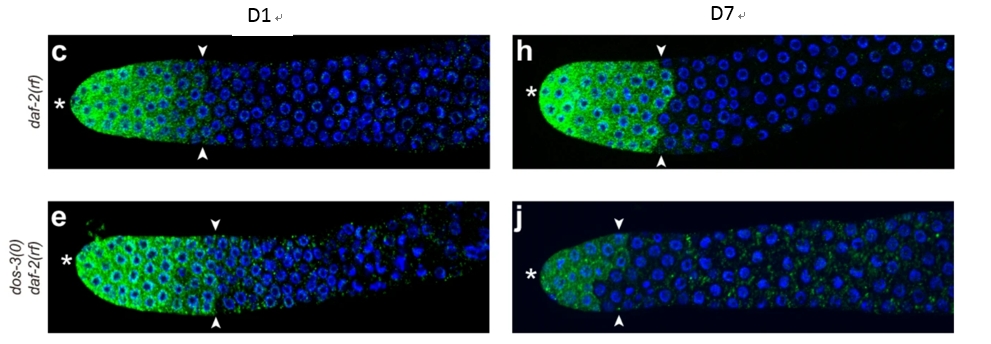
Figure 4: Notch signaling was significantly attenuated in dos-3(0); daf-2(rf) mutants compared to daf-2(rf)
At last, the study’s findings extend beyond the model organism C. elegans. The discovery that human homologs of DOS-3 can substitute for its function in maintaining GSPCs suggests that similar mechanisms may operate in human stem cell systems. This opens the door for developing therapies aimed at preserving stem cell function in aging tissues, enhancing tissue regeneration, and combating age-related degenerative diseases.
The expression of human DLK1, a homologous protein to DOS-3, in C. elegans rescued the age-related loss of GSPCs, demonstrating the potential for translational applications in human health.
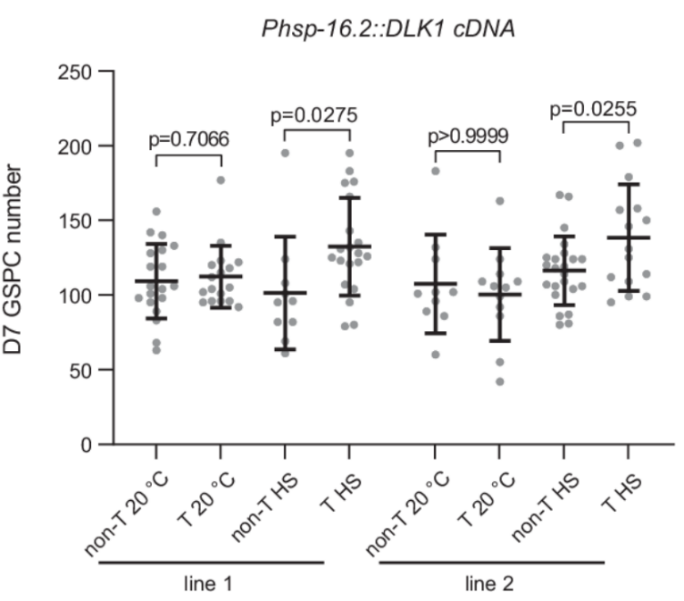
Figure 5: The expression of human DLK1, a homologous protein to DOS-3, in C. elegans rescued the age-related loss of GSPCs
Conclusion
This groundbreaking research provides critical insights into the mechanisms of stem cell aging. By identifying DOS-3 as a mediator of DAF-16/FOXO activity and elucidating its role in activating Notch signaling, the study opens new avenues for potential therapeutic strategies to enhance healthy aging and combat age-related diseases.
Reference: Zhang, Zhifei, Haiyan Yang, Lei Fang, et al. "DOS-3 mediates cell-non-autonomous DAF-16/FOXO activity in antagonizing age-related loss of C. elegans germline stem/progenitor cells." Nature Communications 15 (2024): 4904. doi:10.1038/s41467-024-49318-6







