How Ionizing Radiation Impacts the Nervous System: Insights from C. elegans and the Role of crm-1
2025-09-29 13:46
Keywords
Behavior, Degeneration, Disorder, Dysfunction, Nerve injury, Nervous system, Neurodevelopment, Neuron, Radiation
Contribution of SunyBiotech
SunyBiotech is very honored to provide strain construction services for this study. Strains PHX5872 crm-1(syb5872[crm-1::GFP])V was created for this work by SunyBiotech Corporation.
Introduction
Ionizing radiation is widely used in medicine, industry, and research, but its biological side effects remain a pressing concern. While radiation is well known to damage DNA and hematopoietic systems, its impact on the nervous system is less understood. Clinical reports show that even low doses of radiation can lead to neurological complications, including cognitive decline, psychiatric disorders, and motor dysfunction. However, the molecular pathways underlying these effects are poorly defined.
A study previously published in Neural Regeneration Research explored these questions using C. elegans as a model system. With its well-mapped nervous system and conserved molecular pathways, C. elegans offers a powerful platform for uncovering genetic regulators of neuronal resilience. The researchers focused on how ionizing radiation affects behavior, dopaminergic neuron integrity, and gene regulation, with particular attention to the little-studied gene crm-1 in C. elegans.
Radiation-Induced Dysfunction in C. elegans
To probe the nervous system effects of radiation, C. elegans larvae were exposed to 75 Gy of 60Co γ-radiation. Following irradiation, the animals displayed significant impairments in locomotion and sensory behaviors:
l Head thrashes, a motor output dependent on A- and B-type motor neurons, decreased by about 8% from 109.83 times to 101.07 times per minute (Figure 1A).
l Touch avoidance, a key defensive reflex, dropped by nearly 23%(Figure 1B).
l Foraging ability, which integrates sensory and motor coordination, was significantly lessened in the radiation group by ~89%, 83%, 71%, 70%, and 47% at 2, 4, 6, 8, and 24 hours compared with the control group (Figure 1C).
These results demonstrated that radiation perturbs both motor and sensory circuits in the worm.
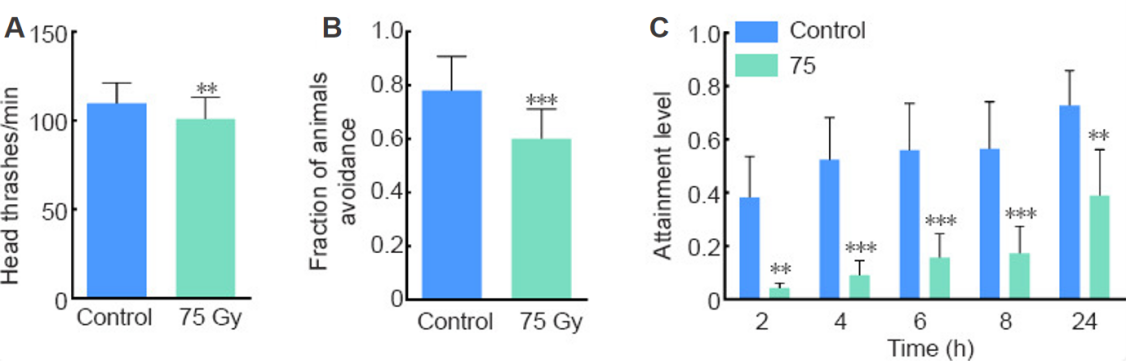
Figure 1. The behavioral effects of ionizing radiation on C. elegans.
Microscopic analysis of transgenic worms expressing GFP in dopamine neurons revealed further insight. After irradiation, cephalic (CEP) and anterior deirid (ADE) dopaminergic neurons (Figure 2A-C, dopamine neuron expression in the head) exhibited reduced fluorescence intensity and fragmented dendrites (Figure 2D-I), evidence of neuronal atrophy and degeneration. Since dopamine signaling is critical for locomotion and learning in both worms and humans, this degeneration provides a likely cellular basis for the observed behavioral deficits.
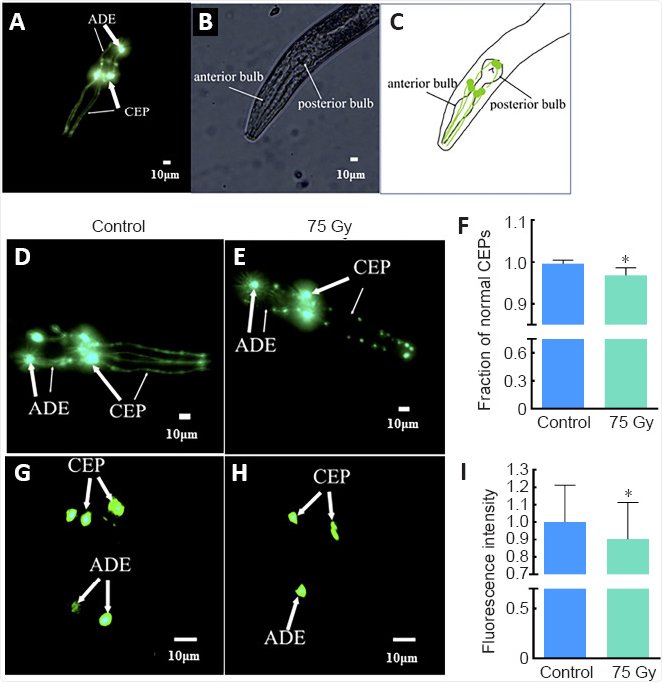
Figure 2. Visualization of dopamine neurons in the head neurons of C. elegans and the effects of ionizing radiation.
crm-1 and nhr-76: Candidate genes related to nervous system functions
To uncover molecular mediators of radiation-induced dysfunction, the authors performed transcriptome analysis (RNA-seq) on irradiated worms. Compared with the control group, 1338 genes were significantly different after radiation. Of these, 638 genes were up-regulated, and 700 genes were down-regulated. Bioinformatics highlighted two genes of interest, both significantly upregulated after radiation:
l crm-1, the human homolog of crm-1 is cysteine rich transmembrane bone morphogenetic protein (BMP) regulator 1 (CRIM1), which is involved in the formation of motor neurons and the regulation of growth and development.
l nhr-76, the human homolog of nhr-76 is peroxisome proliferator activated receptor delta (PPARD), a nuclear receptor that regulates neurogenesis, homeostasis, and fatty acid metabolism in mammals and in C. elegans.
The role of crm-1 in ionizing radiation-induced nervous system dysfunction in C. elegans
Using RNA interference (RNAi), the researchers suppressed crm-1 expression. Four experimental groups were set up: the control group, the crm-1 RNAi group, the radiation group, and the crm-1 RNAi and radiation group. The head thrash frequency (Figure 3A) and the proportion of touch avoidance (Figure 3B) were increased by about 12% from 94.17 times to 105.63 times per minute and 24%, respectively in the crm-1 RNAi and radiation group when compared with the radiation group. However, foraging behavior did not recover (Figure 3C), suggesting that crm-1 selectively regulates certain neural circuits. Importantly, there was no significant difference between the control and crm-1 RNAi group, indicating its pathological role emerges specifically under stress.
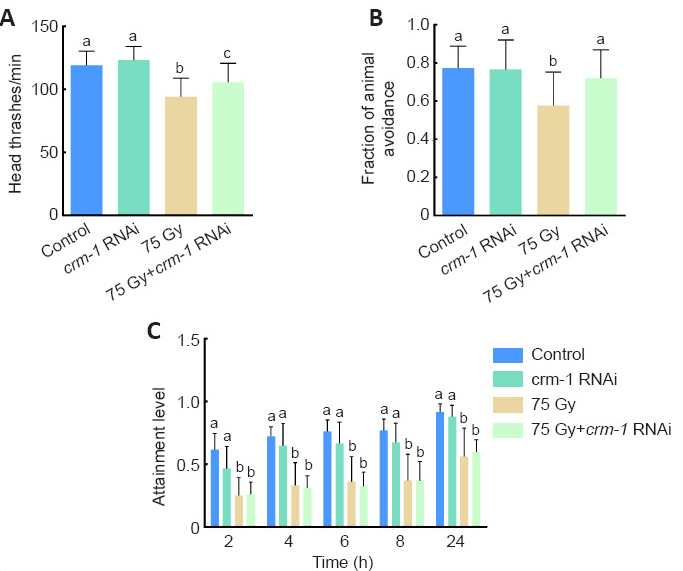
Figure 3. The effects of knockdown of crm-1 gene expression on behavior of C. elegans.
The nhr-76/crm-1 Regulatory Pathway
The study further dissected the relationship between nhr-76 and crm-1. RNAi technology was used to knockdown crm-1 and nhr-76 in wild-type N2 strains (Figure 4A). Silencing nhr-76 reduced crm-1 expression by ~59% (Figure 4C), while crm-1 knockdown did not alter nhr-76 levels (Figure 4B). Therefore, nhr-76 appears to act upstream of crm-1 at the gene level.
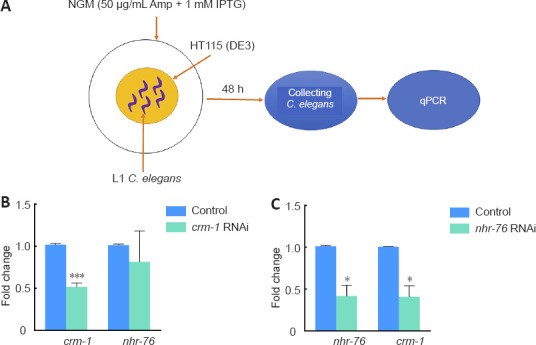
Figure 4. Interaction and RNAi efficiency between crm-1 and nhr-76 in C. elegans.
This genetic hierarchy was validated using a fluorescent crm-1 reporter strain (PHX5872). The fluorescence intensity of the transgenic strain increased by 35% after irradiation when compared with the control group (Figure 5A-B). This suggests that crm-1 expression is increased by irradiation. Conversely, the fluorescence intensity weakened by 36% compared with the control group after knocking down the expression level of the nhr-76 gene (Figure 5C-D). This provides further evidence that crm-1 is regulated by nhr-76.
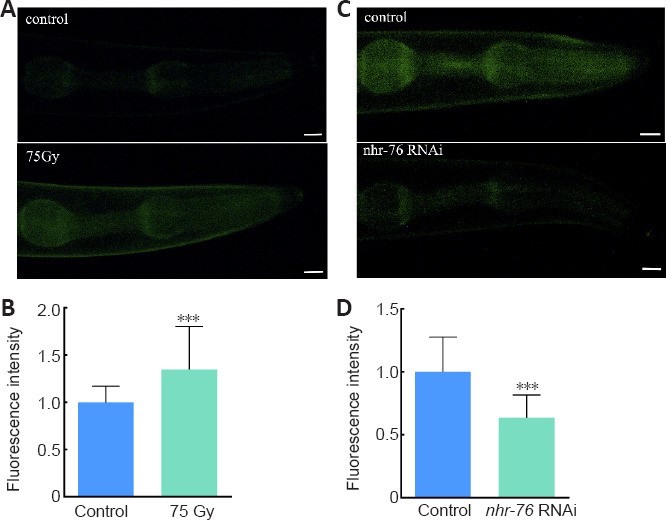
Figure 5. CRM-1 fluorescence intensity changes after irradiation or knockdown of nhr-76 in transgenic strain PHX5872 crm-1(syb5872).
Conclusion
Exposure to ionizing radiation impairs C. elegans behavior, causes dopaminergic neuron degeneration, and triggers upregulation of crm-1 and nhr-76. Suppressing crm-1 alleviates key behavioral deficits, while genetic analysis places crm-1 under the control of nhr-76. This identifies the nhr-76/crm-1 axis as a central mediator of radiation-induced nervous system dysfunction.
By bridging behavioral neuroscience with molecular genetics, this study highlights crm-1 as a promising candidate for future interventions aimed at protecting the nervous system from radiation damage. Beyond its implications for radiation biology, the work underscores the continuing value of C. elegans in unraveling conserved mechanisms of neuroprotection.
Reference
Long HQ, Gao J, He SQ, et al. The role of crm-1 in ionizing radiation-induced nervous system dysfunction in Caenorhabditis elegans. Neural Regen Res. 2023;18(6):1386-1392.
doi:10.4103/1673-5374.357908







