The Germline's Role in Mitokine Signaling: Unraveling Inter-tissue Communication
2024-08-30 17:47
Introduction
Mitochondria are well-known as the energy producers of the cell, but their role in inter-tissue communication is equally vital, particularly under stress. The mitochondrial unfolded protein response (UPRMT) is a protective mechanism activated in response to mitochondrial stress. While neurons have been traditionally recognized as the key players in coordinating these stress signals, recent research by Shen et al. (2024) has revealed that the germline, a tissue previously thought to be functionally isolated from the soma, plays a critical role in this process. This study explores how germline mitochondria influence the signaling between neurons and peripheral tissues like the intestine, providing new insights into mitochondrial health and organismal longevity.
Contribution of SunyBiotech:
SunyBiotech played a critical role in supporting this research by creating specific C. elegans strains that were essential for exploring the function of the ucr-2 gene family in mitokine signaling:
PHX6175: ucr-2.3(syb6175) (3x HA tag)
PHX4838: ucr-2.1(syb4838) [ucr-2.1p::mCherry::GSG::F2A::ucr-2.1]
PHX2912: ucr-2.2(syb2912) [ucr-2.2p::mCherry::GSG::F2A::ucr-2.2]
PHX3476: ucr-2.3(syb3476) [ucr-2.3p::mCherry::GSG::F2A::ucr-2.3]
The Study: Objectives and Key Findings
The primary objective of the study was to identify the mechanisms by which germline mitochondria contribute to inter-tissue UPRMT signaling, with a focus on the ucr-2.3 gene in C. elegans. The study followed a systematic approach to uncover the role of ucr-2.3 and its impact on mitochondrial communication between neurons and the intestine.
The researchers initiated their investigation with a genome-wide mutagenesis screen, which led to the identification of ucr-2.3 as a key gene required for neuron-to-intestine UPRMT signaling. The data revealed that ucr-2.3 is essential for this communication, as loss of this gene significantly suppressed UPRMT activation in the intestine. This was evidenced by fluorescence imaging data presented in Figure 1, where the intestinal UPRMT signal was notably diminished in ucr-2.3 mutants.
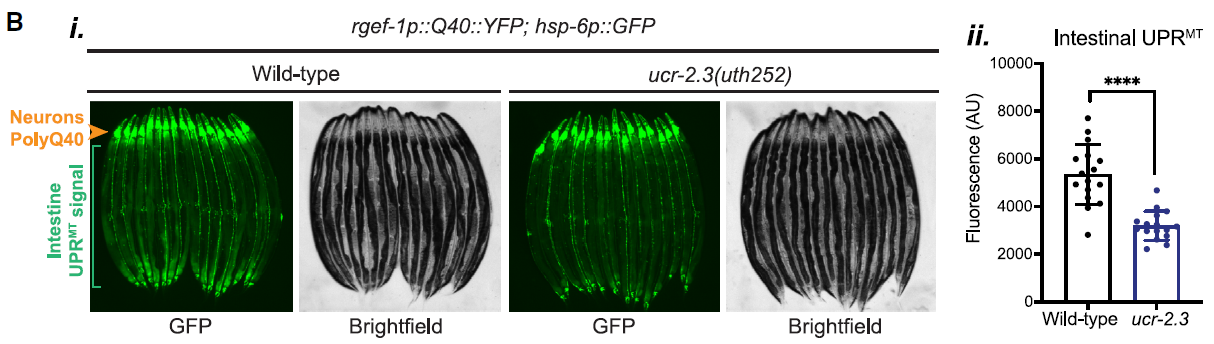
Figure 1: Loss of ucr-2.3 significantly suppresses UPRMT activation in the intestine
Further exploration into the tissue-specific roles of ucr-2.3 involved rescuing its expression in either neurons or the germline of mutant C. elegans. The results, depicted in Figure 2, showed that only the germline-specific expression of ucr-2.3 restored UPRMT signaling. This finding was pivotal, as it challenged the traditional view that the germline is insulated from somatic functions and highlighted its active role in coordinating stress responses across tissues.
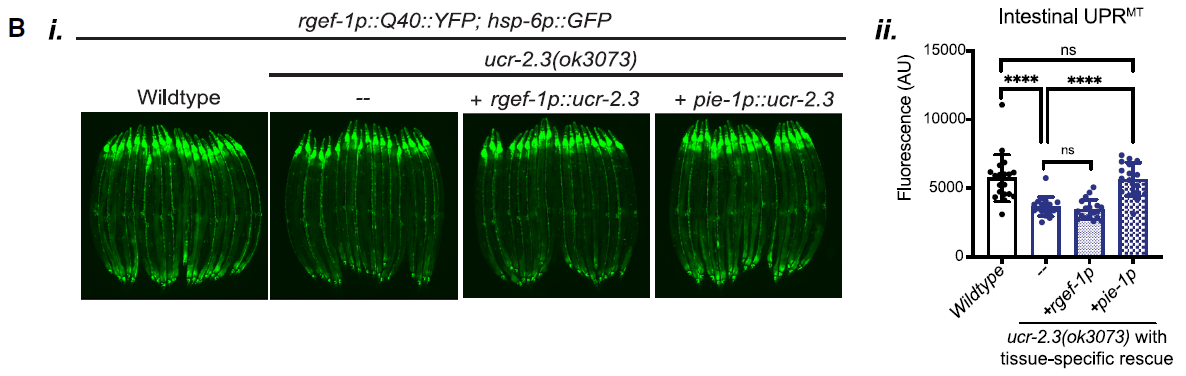
Figure 2: Germline-specific expression of ucr-2.3 restores UPRMT signaling
The study also examined the impact of ucr-2.3 on mitochondrial integrity within the germline. In ucr-2.3 mutants, the mitochondria were found to be fragmented and sparse, as shown in Figure 3. This compromised mitochondrial structure correlated with significant defects in UPRMT signaling, underscoring the importance of ucr-2.3 in maintaining mitochondrial function in the germline.
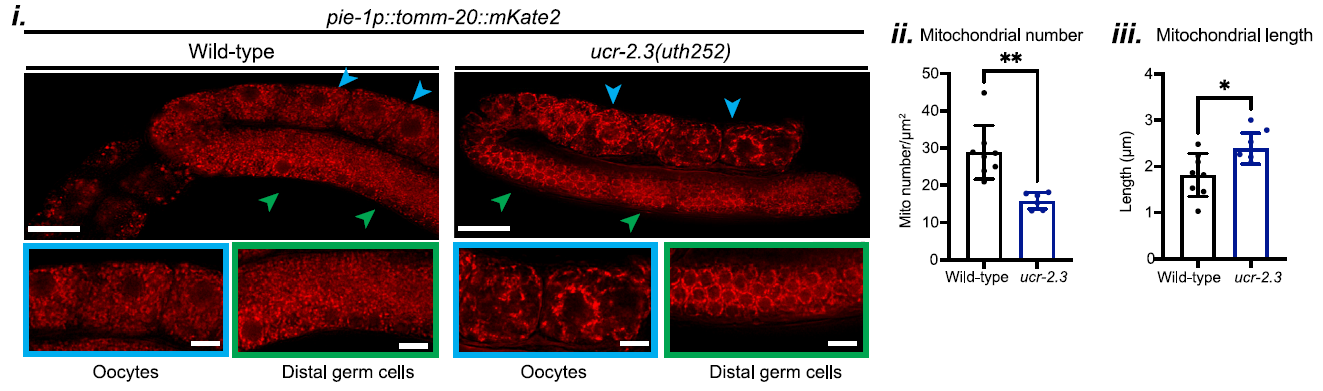
Figure 3: Mitochondria in ucr-2.3 mutants were found to be fragmented and sparse.
Additionally, the researchers uncovered a link between germline mitochondrial function and lipid metabolism. In ucr-2.3 mutants, there was a marked increase in intestinal lipid content, demonstrated by the data in Figure 4. This elevated lipid level was associated with the suppression of UPRMT signaling, suggesting that proper lipid metabolism, regulated by germline mitochondria, is crucial for the activation of mitochondrial stress responses in peripheral tissues.
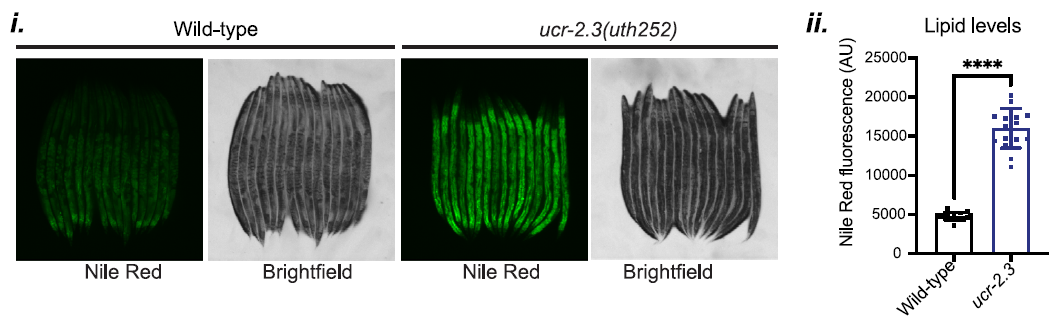
Figure 4: ucr-2.3 mutants show a marked increase in intestinal lipid content
The suppression of UPRMT was further confirmed through RNAi knockdowns targeting lipid metabolic genes, where the knockdown of fat-2 rescued UPRMT signaling in the ucr-2.3 mutants, as illustrated in Figure 5.
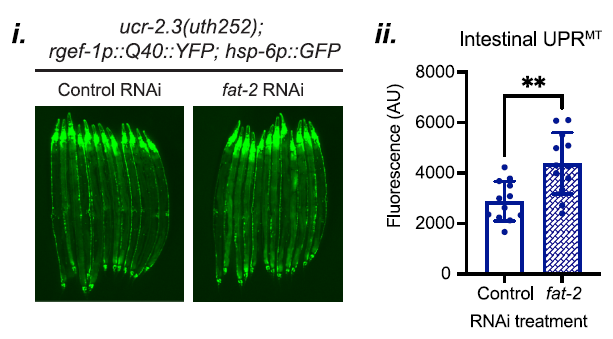
Figure 5: Knockdown of fat-2 rescues UPRMT signaling in ucr-2.3 mutants
These findings led the authors to propose the "Parallel Signaling" model. In this model, the germline and neuronal signals work in tandem to regulate UPRMT activation in the intestine, ensuring that mitochondrial stress responses are managed efficiently across the organism. The model is supported by the integration of the data presented, which collectively illustrates how the germline's influence on lipid metabolism and mitochondrial integrity plays a critical role in maintaining overall cellular health and preventing dysfunction.
Conclusion
Shen et al.'s study provides compelling evidence that the germline, through the ucr-2.3 gene, plays a critical role in coordinating mitochondrial stress responses between neurons and peripheral tissues like the intestine. The data strongly support the conclusion that germline mitochondria are integral to maintaining mitochondrial health and inter-tissue communication. This research not only challenges the existing paradigms of germline insulation from somatic functions but also offers a new framework for understanding the role of mitochondria in aging and disease.
References
Shen, K., Durieux, J., Mena, C. G., et al. (2024). The germline coordinates mitokine signaling. Cell, 187, 1–16. https://doi.org/10.1016/j.cell.2024.06.010







